
Redox Flow Batteries
Redox flow batteries (RFBs) are promising technologies for large scale electricity storage, owing to its design flexibility in decoupling power and energy capacity. Our research focuses on materials and device design to increase the energy density and reduce the cost of RFBs to improve its competitiveness for both stationary and transportation applications.
-
Multiple redox semi-solid-liquid sulfur-based flow catholyte:
(a) Sulfur-impregnated carbon (S/C) flow catholyte: 1
We report a new flow cathode that consists of sulfur-impregnated carbon (S/C) suspended in the electrolyte, which offers high volumetric capacity and reduces the interfacial resistance between the insulating active materials and conductive carbon network compared to Li-ion flow batteries (Fig. 1a). This design achieved a high catholyte capacity 294 Ah/L with long cycle life (>100 cycles), high columbic efficiency (>90%) and energy efficiency (82%).1 Pseudo-in situ electrochemical impedance spectroscopy and scanning electron microscopy revealed superior electrochemical and morphological reversibility of the S/C catholyte. Our concept of impregnating insulating solid active materials with conductive carbon network offers a promising direction to further increase the energy density and cycling reversibility of redox flow batteries and semi-solid flow batteries.
(b) New concept of multiple redox semi-solid-liquid:
Building on the first work, we develop Multiple Redox Semi-Solid-Liquid (MRSSL) flow catholyte that takes advantage of both highly soluble active materials in the liquid phase and high-capacity active materials in the solid phase, to form a biphase MRSSL catholyte (Fig. 1b).2 We used liquid lithium iodide (LiI) electrolyte and solid S/C composite as an example to demonstrate a LiI-S/C MRSSL catholyte, which achieved the highest catholyte volumetric capacity (550 Ah/Lcatholyte) to date with superior energy density (580 Wh/Lcatholyte+lithium) with high columbic efficiency (>95%). We further show that the presence of LiI synergistically facilitates the electrochemical utilization of sulfur and reduces the viscosity of the catholyte. The MRSSL concept creates wide-open opportunities for numerous combinations of solid and liquid active materials for next-generation high-energy-density energy storage applications.

-
Highly-concentrated all-liquid redox-active flow electrolyte:
(a) Low-melting-point Ferrocene derivative flow catholyte: 3
We develop a high-energy-density non-aqueous RFB based on a low-melting-point (37-40°C) ferrocene derivative, 1, 1-dimethylferrocene (DMFc) operated at its liquid state (Fig. 2a).3 The liquid redox-active DMFc not only contributes to high capacity but also acts as a solvating medium to the ion-conducting salts. By replacing the redox-inactive solvent with redox active liquid DMFc at 50 °C, we successfully demonstrated a highly concentrated catholyte and achieved a volumetric density of ~68 Ah/Lcatholyte, with stable life. (Fig. 2b). Our work demonstrates that exploiting low-melting-point redox-active species at its melting state is a promising direction to develop high-energy-density RFBs.

(b) Highly-Concentrated Aqueous Redox Flow Chemistry:
Two examples of high-concentrated aqueous redox flow chemistry developed in our group:
(i) Zinc/iodine-bromide (I2Br ) battery (ZIBB)
Highly soluble iodide/triiodide (I /I ) couple is one of the most promising redox-active species for RFBs. However, to ensure high reversibility, only two-third of the iodide capacity is accessed and one-third of the iodide ions are acting as the complexing agent to stabilize the iodine (I2) forming I (I2I ). We propose to use bromide ions (Br ) as the complexing agent to stabilize the free iodine, forming iodine-bromide ions (I2Br ), thereby freeing up the capacity of the iodide ions (Fig. 3a).4 We show that the utilization of iodide increases in the presence of Br ions without involving redox reactions of Br /Br . With this strategy, we demonstrate a novel zinc/iodine-bromide (I2Br ) battery (ZIBB) achieving an energy density of 101 Wh/Lposolyte+negolyte, which is the highest energy density achieved for aqueous flow batteries to date (Fig. 3b). This strategy can be further applied to halide systems in nonaqueous media, forming a Li/I2Br battery (LIBB). We use Electrospray Ionization Mass Spectrometry and identify the presence of the I2Br of the posolytes, supporting that the Br ions serve as complexing agent to stabilize iodine ions. Our strategy enables high-energy-density iodide-based energy storage with stable cyclability and high efficiencies.
-
- 3-
3- -
-
-
-
-
- 3-
-
-
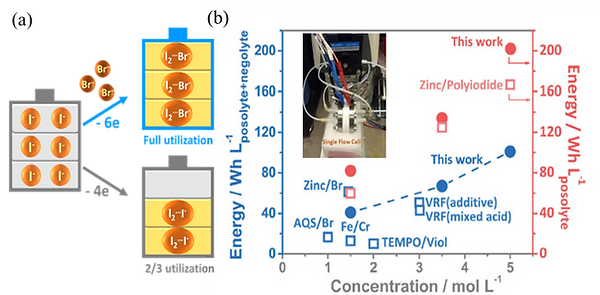
Fig. 3. (a) Design concept of bromide as the complexing agent to stabilize iodine4 (b) Comparison of the demonstrated energy density of ZIBB and state-of-the-art aqueous flow batteries.4
(ii) Polysulfide/iodide battery (PSIB).
To address the high cost of vanadium, we employ highly soluble, inexpensive and reversible polysulfide and iodide species to demonstrate a high-energy and low-cost all-liquid polysulfide/iodide redox flow battery (PSIB). In contrast to metal-hybrid or semi-solid approaches that are usually adapted for high-energy RFBs, the all-liquid characteristic of the PSIB is crucial to enable practical scale-up development. Combining the achieved energy density (43.1 W h/L Catholyte+Anolyte) and the inherent low materials cost of sulfur and iodine compared to vanadium, the PSIB system demonstrates a significantly lower materials cost per kilowatt hour ($85.4 kW/h) compared to the state-of-the-art vanadium based redox flow batteries ($152.0–154.6 kW/h). Operando UV-Visible spectroscopy reveals superior electrochemical reversibility of both electrolytes. With its demonstrated energy density, inherent low material cost and benign chemical natures, the all-liquid PSIB offers a promising solution for high-energy-density and low-cost energy storage applications.
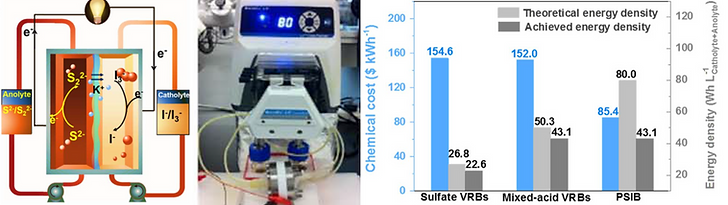
Fig. 4. A schematic and a photograph of the PSIB flow cell. Chemical cost and volumetric energy analysis.5
Reference:
1 Chen H., Zou Q., Liang Z., Liu H., Li Q., and Lu Y.C.*, "Sulphur-Impregnated Flow Cathode to Enable High-Energy-Density Lithium Flow Batteries" Nature Communications, 6, Article number: 5877, (2015). Link
2 Chen H., and Lu Y.C.*, "A High-Energy-Density Multiple Redox Semi-Solid-Liquid Flow Battery" Advanced Energy Materials, 2016, 6, 1502183, DOI: 10.1002/aenm.201502183. Link
3 Cong G., Zhou Y., Li Z., and Lu Y.C.*, " A Highly Concentrated Catholyte Enabled by a Low-Melting-Point Ferrocene Derivative" ACS Energy Letter, 2017, 2, pp 869–875 Link
4 Weng G.M., Li Z., Zhou Y., Cong G., and Lu Y.C.*, "Unlocking the capacity of iodide for high-energy-density zinc/polyiodide and lithium/polyiodide redox flow batteries" Energy & Environmental Science, 2017,10, 735-741 Link
5 Li Z., Weng G.M., Zou Q., Cong G., and Lu Y.C.*, "A High-Energy and Low-Cost Polysulfide/Iodide Redox Flow Battery" Nano Energy, 30, 283-292, 2016 Link